|
Singapore-MIT Alliance for Research & Technology |
||||||||||||
|
|
Research Projects Under Thrust 2: Multifunctional cytometryJongyoon HAN (MIT), Linda GRIFFITH (MIT), Krystyn VAN VLIET (MIT), Mary CHAN (NTU), Chia Hung CHEN (NUS), Peter CHEN (NUS), Sang Ho KIM (NUS), C.T.LIM (NUS), Darren LIM (NCC), Peter PREISER (NTU), Martin PUMERA (NTU), Bruce RUSSELL (A*STAR-SIgN & NUS), Lam YEE Cheong (NTU), G.V.Shivashankar (MBI/NUS) In Thrust 2, we develop novel functional cell assays and cell manipulation methodologies, largely relying on physical or biological / physiological characteristics of cells that provide advantages for more accurate and faster means to isolate and understand the function of rare cells within body fluids and tissues. Specific projects include development of microfluidic magnetic resonance measurements to study redox/metabolomic states of cells noninvasively; integrated electrochemical and deformability measurements of cells to elucidate complicated redox changes occurring in blood and other tissues; microfluidic and mechanical cell sorting platforms applicable to clinical challenges in rare-cell-sorting, including isolation of circulating tumor cells; and microfabricated in situ cloning assays that include localized sensors of cellular function at all culture stages for rare and mixed cell types.1. High Throughput Pathogen Separation Using Microfluidics (Jay Han (MIT/SMART), Chwee Teck Lim (NUS), Ali Asgar S.Bhagat (SMART-BioSyM), Han Wei Hou (NUS/SMART/MIT),Leon D.Li (MIT))
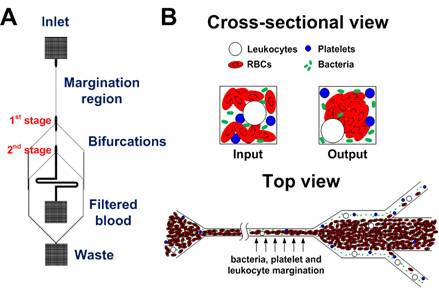
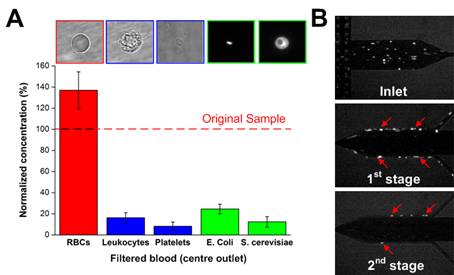
Sepsis is an adverse systemic inflammatory response caused by microbial infection in blood. In this work, we develop a simple microfluidic approach for intrinsic, non-specific removal of both microbes and inflammatory cellular components (platelets and leukocytes) from whole blood, inspired by the in vivo phenomenon of leukocyte margination. As blood flows through a narrow microchannel (20 × 20 µm), deformable red blood cells (RBCs) migrate axially to the channel centre, resulting in margination of other cell types (bacteria, platelets and leukocytes) towards the channel sides (Figure 26). With use of a simple cascaded channel design, the blood samples undergo a 2-stage bacteria removal in a single pass through the device, thereby allowing higher bacterial removal efficiency. As an application for sepsis treatment, we demonstrated separation of Escherichia coli and Saccharomyces cerevisiae spiked into whole blood, achieving high removal efficiencies of ~80% and ~90%, respectively (Figure 27). Inflammatory cellular components were also depleted by >80% in the filtered blood samples, which could help to modulate the host inflammatory response and potentially serve as a blood-cleansing method for sepsis treatment. The developed technique offers significant advantages including high throughput (~1mL/hr per channel) and label-free separation that allows non-specific removal of any blood-borne pathogens (bacteria and fungi). The continuous processing and collection mode potentially enables the return of filtered blood to the patient directly, similar to a simple and complete dialysis circuit setup. Due to design simplicity, further multiplexing is possible by increasing channel parallelization or device stacking to achieve higher throughput comparable to convectional blood dialysis systems used in clinical settings.
Selected publications
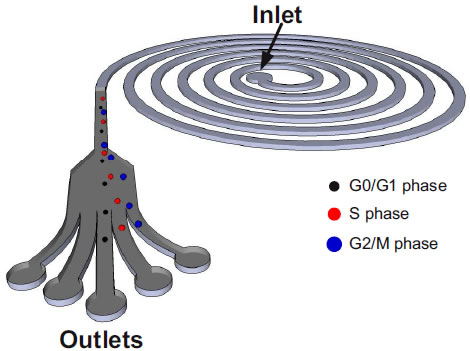
2. Pressure actuated micro-valve system for active sorting of cells by stiffness(Jay Han (MIT/SMART-BioSyM), Peter Chen (NUS), Peng Weng Kung (SMART-BioSyM), Guan Guofeng (NUS/SMART-BioSyM)Then goal of this project is to build a cell sorting system with an active micro-valve to seperate infected rigid red blood cells from healthy ones. We have developed technologies for fabrication of channels and membrane, setup of air actuated valve with DAQ and VI software, image acquisition & image processing for the cell recognition. 3. Microfluidics based sorting of mesenchymal stem cells (MSCs)(Jay Han (MIT/SMART-BioSyM), Chwee Teck Lim (NUS), Krystyn Van Vliet (MIT/SMART-BioSyM), Ali Asgar Bhagat (SMART-BioSyM), Shi Hui (SMART-BioSyM) and Jacky Lee Wong Cheng (NUS/SMART-BioSyM)Cell cycle synchronization is of paramount importance for studying cellular properties and biological processes involved in various stages of the cell cycle. BioSyM researchers have developed a microfluidics based approach to synchronize the cell cycle of a primary –cells, human bone marrow-derived mesenchymal stem cells (hMSCs), using inertial forces in spiral microchannels. The device operating principle exploits the relationship between the volume (and thus diameter) of a cell and its phase in the cell cycle, in order to fractionate hMSCs populations into synchronized sub-populations enriched in cell cycle fractions of G0/G1, S and G2/M phases
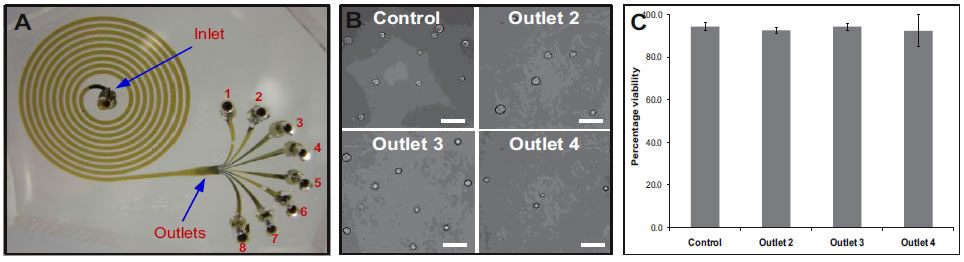
A. Photograph of the spiral microchannel with one inlet and eight outlets fabricated in PDMS. B. Optical micrographs of the size sorted cells collected from outlets 2, 3 and 4. bar = 50μm. C. Viability of sorted hMSCs verified using trypan blue exclusion assay. Results indicate that the shear experienced at very high flow rates (2 mL/min), do not compromise the cells achieving >90% cell recovery. Selected publications
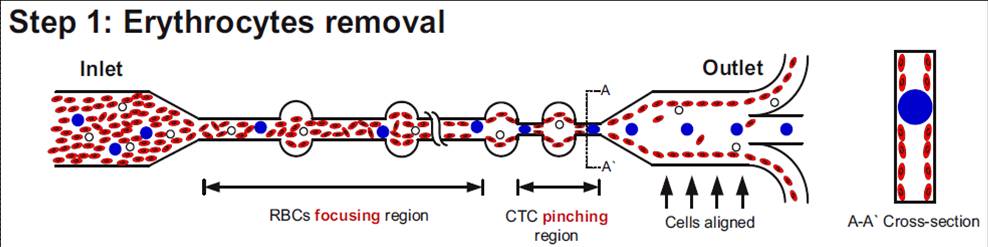
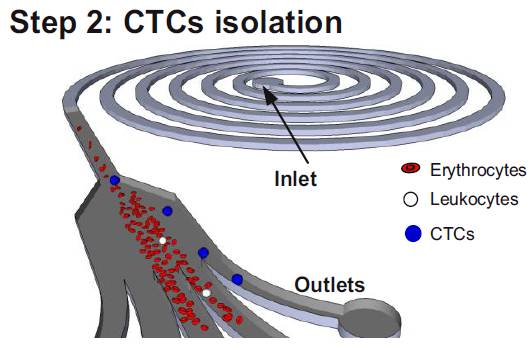
4. Microfluidics based isolation of circulating tumor cells(Jay Han (MIT/SMART-BioSyM), Chwee Teck Lim (NUS), Ali Asgar S.Bhagat (SMART-BioSyM), Han Wei Hou (NUS/SMART-BioSyM)The goal of this project is to develop microfluidic devices for isolation of rare circulating tumor cells from blood. We developed a novel technique which takes advantage of the preferential cell focusing in high aspect-ratio microchannels coupled with pinched flow dynamics for isolating low abundance cells from blood. We have demonstrated isolation of circulating tumor cells (CTCs) from blood exploiting the difference in size between CTCs and hematologic cells.
Schematic illustrating the erythrocyte focusing process in an high aspect ratio rectangular microchannel. The shear modulated inertial lift forces efficiently equilibrates all the cells along the channel side walls and are removed from the two side outlets. The channel design also consists of wider circular sections placed at regular intervals yielding higher channel structural integrity (preventing channel collapse due to high aspect ratio). By patterning a pinched region prior to the outlet, the larger CTCs are collected through the center outlet. (Step 2) Schematic of the spiral microfluidic design developed to isolate CTCs from blood. Under the influence of inertial lift forces and Dean drag force, the erythrocyte depleted blood sample (< 0.01% hematocrit) consisting of leukocytes and CTCs are size fractionated to obtain pure population of rare CTCs for cancer diagnosis. Selected publications
5. Deformability Cell Cytometry(Jay Han (MIT/SMART-BioSyM), Ming Dao (MIT/SMART-ID), Hansen Bow (MIT) and Sha Huang (MIT)The goal of this project is to develop microfluidic devices for quantitatively measure the cell deformability / stiffness, as a scientific tool to study biomechanical properties of cells in a high throughput manner. Here we introduce an automated microfabricated “deformability cytometer” that measures dynamic mechanical responses of 1000–10000 individual RBCs in a cell population. Fluorescence measurements of each RBC are simultaneously acquired, resulting in a population-based correlation between biochemical properties, such as cell surface markers and dynamic mechanical deformability. This device is especially applicable to heterogeneous cell populations. We demonstrate its ability to mechanically characterize a small number of P. falciparum-infected (ring stage) RBCs in a large population of uninfected RBCs. Selected publications
6. On-Chip Magnetic Resonance for single cell level detection(Peng Weng Kung (SMART-BioSyM), Jay Han (MIT/SMART-BioSyM), Nam Trung Nguyen (NTU), Kong Tian Fook (NTU/SMART-BioSyM)Magnetic Resonance (MR) Spectroscopy-Imaging is a mature and developed technology, well-known for its non-invasive analysis. Currently, conventional MR systems often require large sample volumes (in the order of mL) and bulky, expensive, high-field magnets, due to its low signal-to-noise ratio and low sensitivity which are however, not the inherent limitation of MR principle. With the advent of microfabrication and microfluidic technology, miniaturized magnetic resonance (MR) systems have been developed, with much higher sensitivity in terms of sample mass, and comparable sensitivity in terms of target concentration. This was achieved by miniaturizing the conventionally large detection coil to a micro-sized coil (often termed as microcoil) combined with microfluidic sample channels enjoying high filling factor. This project is an attempt to design, fabricate and develop a functional and portable medical diagnostic tool based on the principle of magnetic resonance. 7. Early detection of Endometriosis(Steve Tannenbaum (MIT/SMART-BioSyM & ID), Linda Griffith (MIT/SMART-MIT), Jagath Rajapakse (NTU), Jerry Chan (NUS-Dule/KKH), Lee Yie Hou (SMART-ID & BioSyM), Ng Chee Ping (SMART-BioSyM) and Tan Chin Wen (NUS/SMART-BioSyM)Endometriosis is a pathological condition afflicting 5-10% of women in the general population. It manifests clinically with cyclical debilitating pain, ovarian cysts, pelvic adhesions and have a complex interaction with infertility. Current knowledge of its aetiological origins is poor, and the early events leading to its formation is even more obscure despite decades of research in this field. Consequently, treatment of this condition is empirical, with no novel therapeutics being developed which addresses its aetiological origins. Stopping the disease from establishing is the best way to treat this condition. We integrate tissue engineering principles, novel biosensors, real time analysis and systems biology approaches in delineating the very early events. We build a microfluidic based system with biosensors and real-time imaging capacity to delineate the early events associated with the interactions between endometrial and peritoneal cell types within a complex milieu of inflammatory and immuno-modulatory cell types. We use endometrial tissues from both normal and endometriotic lesions for these assays, and parse out regulatory networks associated with early disease formation. This system will not only provide a mechanistic view of the pathogenesis of endometriotic lesions, but will serve as a system for testing novel therapeutics derived through such testing. In addition, we utilise advance proteomic approaches to derive biomarkers of endometriosis activity which will be of relevance to the follow up of the disease course of endometriosis and its response to therapeutics.This project represents an integrative approach to defining how endometriosis is established through an in vitro system of relevance not only to identifying key regulators of invasion, but also to the development of therapeutics for this common debilitating disease. 8. Microwell Functional Assays for High Content Screening and Analysis of Stem Cell and Endometriotic Cell Colonies(Paula Hammond (MIT/SMART-BioSyM & ID), Linda Griffith (MIT/SMART-MIT), Mary Chan (NTU), Jerry Chan (NUS-Dule/KKH), Ng Chee Ping (SMART-BioSyM), Liu Yunxiao (SMART-BioSyM) and Tan Chin Wen (NUS/SMART-BioSyM)
|
|
||||||||||
BioSyM IRG
Publications Useful Links Global Enterprise for Micro Mechanics and Molecular Medicine (GEM4): www.gem4.org Mechanobiology Institute: http://mbi.nus.edu.sg/ Singapore-MIT Alliance for Research and Technology (SMART): http://smart.mit.edu/home.html
|
||||||||||||